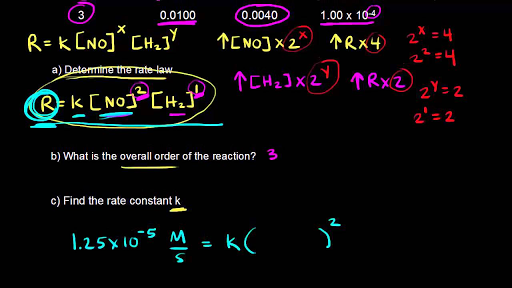What is the order with respect to each reactant and the overall order of the reaction? write the rate law. - Sarthaks eConnect | Largest Online Education Community
![SOLVED: Classify each rate law based on whether the reaction is first-order; second-order; O third-order overall: First-order Second-order Third-order Answer Bank rate k[HCN] rate k[O] [NO] [NzE rate [BFs] [NH,] rate kINO]? SOLVED: Classify each rate law based on whether the reaction is first-order; second-order; O third-order overall: First-order Second-order Third-order Answer Bank rate k[HCN] rate k[O] [NO] [NzE rate [BFs] [NH,] rate kINO]?](https://cdn.numerade.com/ask_previews/7a671ad0-bb32-4650-a5e2-0dc410a20fb1_large.jpg)
SOLVED: Classify each rate law based on whether the reaction is first-order; second-order; O third-order overall: First-order Second-order Third-order Answer Bank rate k[HCN] rate k[O] [NO] [NzE rate [BFs] [NH,] rate kINO]?
![SOLVED: A reaction is third order overall , a possible rate law would be? A- k.[A] B- K.[A].[B]2 C- K.[A].[B] D- K.[A]2 In a zero order reaction , doubling the concentration of SOLVED: A reaction is third order overall , a possible rate law would be? A- k.[A] B- K.[A].[B]2 C- K.[A].[B] D- K.[A]2 In a zero order reaction , doubling the concentration of](https://cdn.numerade.com/ask_previews/7e2ef9eb-0c1a-43c2-bff5-2356d0856e78_large.jpg)
SOLVED: A reaction is third order overall , a possible rate law would be? A- k.[A] B- K.[A].[B]2 C- K.[A].[B] D- K.[A]2 In a zero order reaction , doubling the concentration of

Determine the overall order of reaction to which the following rate law apply Rate=k Show the half-life for - Brainly.com
![SOLVED: What is the overall reaction order for the reaction that has the rate law Rate = k[O2][NO]2? zero order first order second order third order SOLVED: What is the overall reaction order for the reaction that has the rate law Rate = k[O2][NO]2? zero order first order second order third order](https://cdn.numerade.com/ask_previews/bea16924-e043-4592-9ba1-7c64b3c6ae29_large.jpg)
SOLVED: What is the overall reaction order for the reaction that has the rate law Rate = k[O2][NO]2? zero order first order second order third order

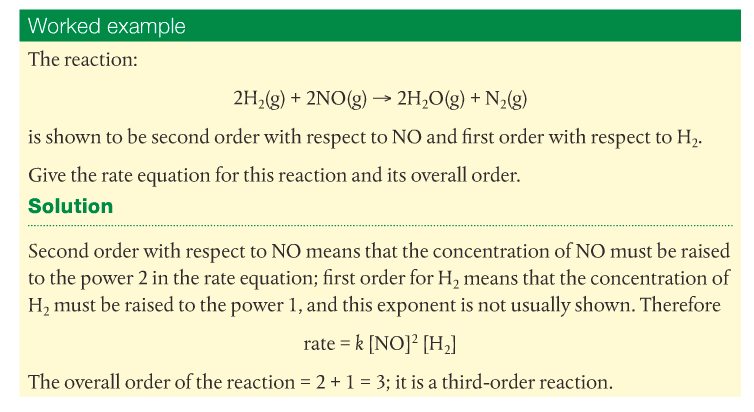
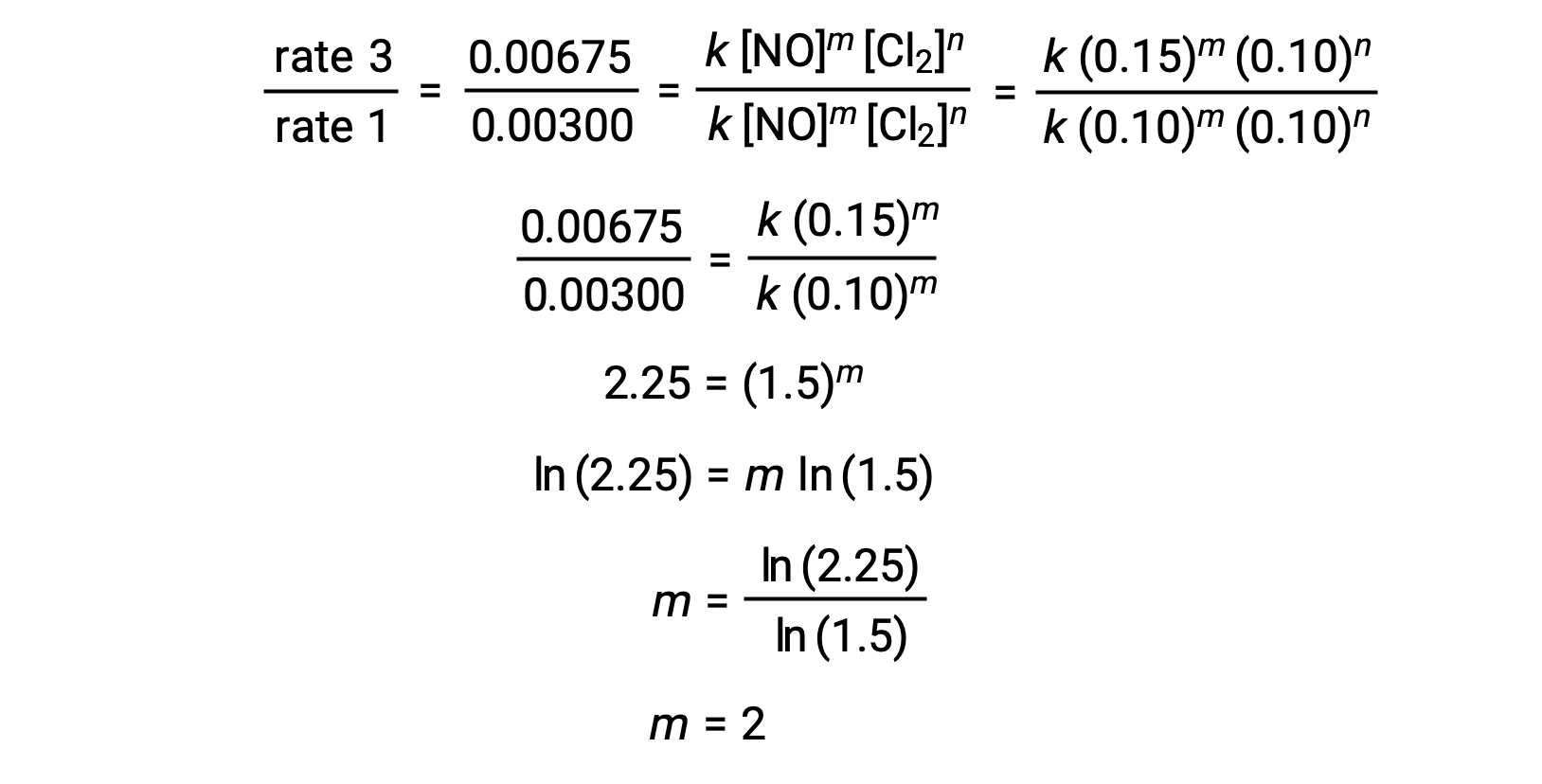
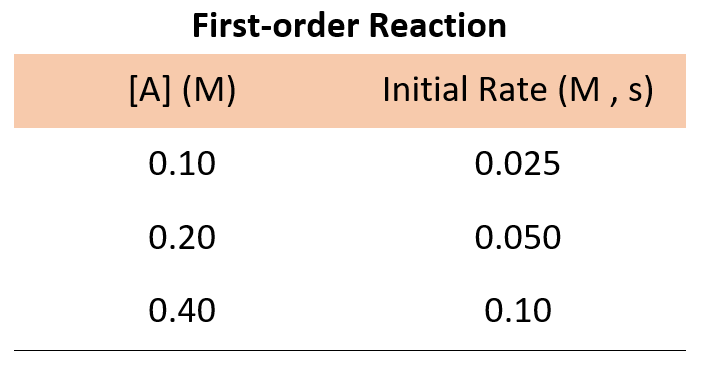
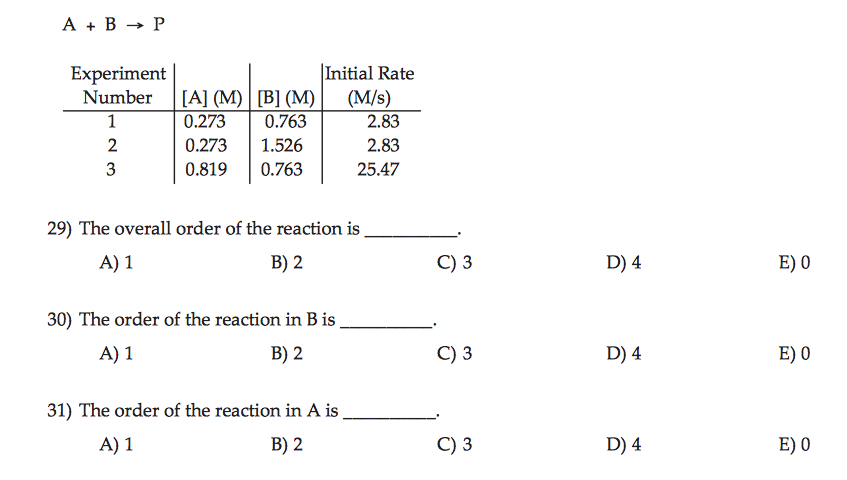
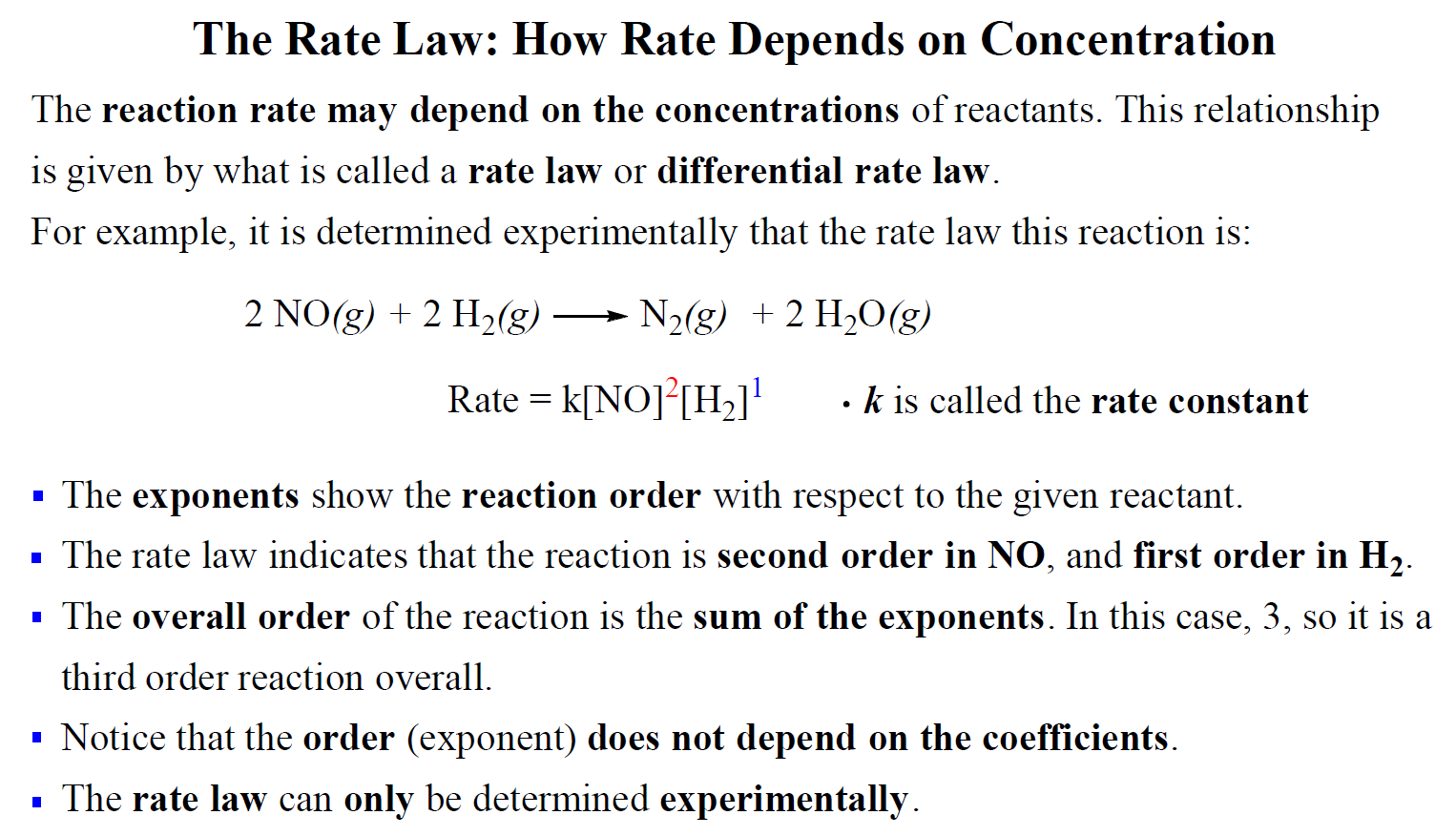
The overall order of the reaction is the sum of the exponents of all the reactants in the rate expression.
![Which of the following rate laws has an overall order of 0.5 for the reaction involving substances x, y and z? ([Cx],[Cy] and [Cz] respectively are the concentrations of x,y and z) Which of the following rate laws has an overall order of 0.5 for the reaction involving substances x, y and z? ([Cx],[Cy] and [Cz] respectively are the concentrations of x,y and z)](https://haygot.s3.amazonaws.com/questions/1958686_661431_ans_54de31a89c134e31baf64d6b1781bccb.jpg)
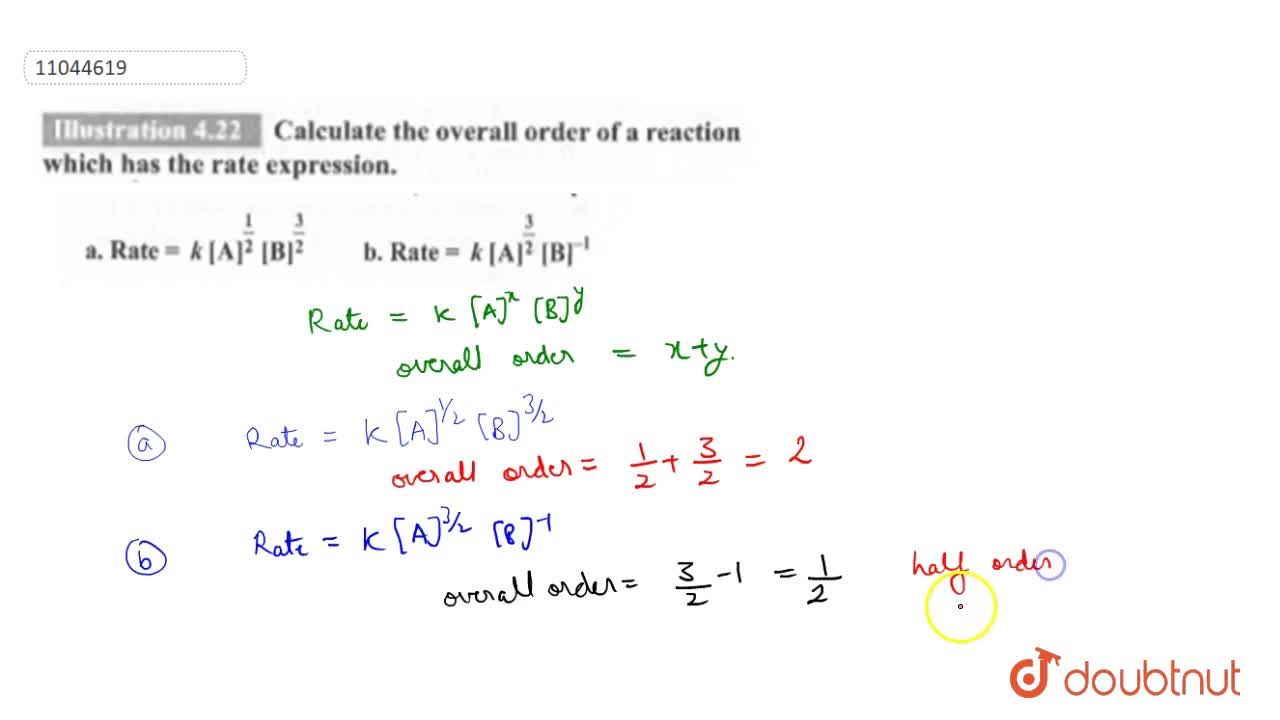



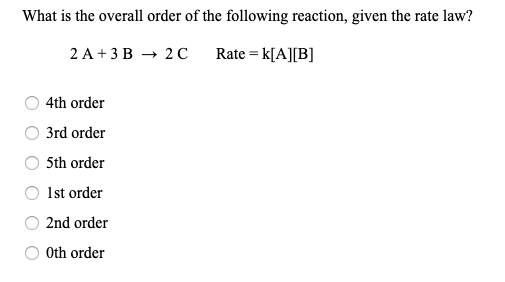







![The form of rate law for a reaction is expressed as, rate = k[Cl(2)][N The form of rate law for a reaction is expressed as, rate = k[Cl(2)][N](https://d10lpgp6xz60nq.cloudfront.net/ss/web-overlay-thumb/648636.webp)